Read time: 6.5 minutes
As a global healthcare leader, McKesson is dedicated to improving the health of patients everywhere. That work shines through every day across all parts of our company, and it’s especially been magnified in the wake of the COVID-19 pandemic.
From day one, McKesson has been fully committed to supporting vaccination efforts. Today, McKesson plays a pivotal role assisting in different aspects of this herculean effort not only in the U.S. and its territories, but throughout Canada and parts of Europe as well. And while each state, province and country may be at different stages of bringing vaccines to everyone who wants one, McKesson is working hand-in-hand with governments, public health agencies, vaccine manufacturers and healthcare providers around the globe to help make this critical undertaking possible.
Whether operating as a centralized distributor, building ancillary supply kits needed to administer the vaccines or making sure shots get into the arms of people, here are several ways we are supporting the world to stop this pandemic once and for all.
Behind Every Package is a Person

As the U.S. government’s centralized distributor for frozen and refrigerated COVID-19 vaccines, we take to heart the job we’ve been asked to do on behalf of the country. In fact, as of April 2, our teams have already shipped more than 100 million vaccine doses across the country.
From the moment we receive vaccine orders from the U.S. government, our teams pack the doses into coolers specially designed to maintain the vaccines’ temperature requirements. And while their role supporting this effort primarily centers around picking, packing and shipping these vaccines to administration sites across the country, the significance of their labor is never lost on them.
At the end of February, the Food & Drug Administration (FDA) signaled a new historic moment for our country when it granted Emergency Use Authorization for the Janssen COVID-19 vaccine from Johnson & Johnson – the first one-shot refrigerated vaccine to receive FDA approval. In a spontaneous moment at our Louisville, Ky. distribution center (DC), our team on the ground commemorated this moment by signing the first box of vaccine doses with words of hope and encouragement for its recipients.
“It was a humbling moment, knowing that we now had the vaccine in our hands and we were able to ship it out and be able to send it to so many people,” says Kristi Larson, an operations supervisor at the Louisville, Ky. DC who joined her colleagues in signing the box. “I think I was crying for half the time.”
Packing the Ancillary Supply Kits

In addition to our work distributing vaccine doses across the country, our Medical-Surgical team has been working since last fall to create ancillary supply kits for healthcare workers administering the vaccines. In fact, our team is ahead of the delivery timeline on the production, storage and shipment of these ancillary supply kits for every type of COVID-19 vaccine authorized for emergency use in the U.S.
Along with the logistical and physical effort involved in picking, packing and shipping the ancillary supply kits, our team also flexed to adapt to the changing needs and opportunities to get more vaccine doses to more Americans. In January 2021, when the FDA approved a label update to the Pfizer COVID-19 vaccine that now recognizes six, rather than five, doses per vial, we began building the new ancillary supply kit within less than a week. This change also meant we had to reconfigure existing ancillary supply kits to support 1,170 total doses per kit from the original 975 doses, which we did in less than one month. Since then, we have produced 110,000 kits to support the six doses per vial of the Pfizer COVID-19 vaccine. And, we’re fully prepared to support the increase in the number of doses per vial for the Moderna COVID-19 vaccine, an increase which the FDA approved on April 1.
Helping Underserved Populations Get Vaccinated
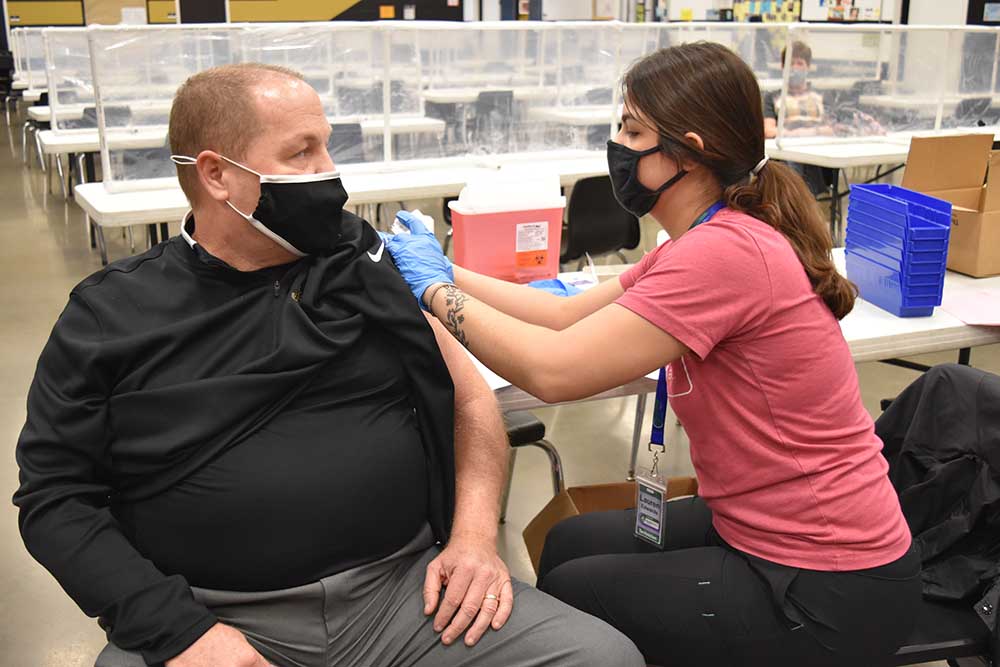
Approximately nine out of 10 Americans live within five miles of a community pharmacy. And because pharmacists are frequently the only healthcare provider available to serve patients living in rural and underserved areas, their role is especially crucial to today’s vaccination efforts.
Since COVID-19 vaccines first started becoming available last December, hundreds of Health Mart pharmacies – a McKesson franchise network of independent, locally owned pharmacies – have been helping administer vaccines in cooperation with state and federal plans. In February, the U.S. government launched the Federal Retail Pharmacy Program for COVID-19 Vaccination to help expand vaccine access to pharmacies across the country. As a partner in the federal program, Health Mart pharmacies began administering vaccines in mid-February at select locations in Kansas, Missouri, Oregon and Washington. And last month, the program expanded to locations in Texas, Mississippi and Iowa. As more vaccine doses become available, additional Health Mart locations across the country stand ready to help their communities overcome the pandemic.
European Efforts Full Steam Ahead

While McKesson is the U.S. government’s centralized distributor for refrigerated and frozen vaccines in the U.S., McKesson Europe is also playing a major role in helping support governments and public health bodies in not only distributing COVID-19 vaccines across several European countries, but administering them in pharmacies as well.
McKesson already delivers to over 50,000 hospitals and pharmacies every single day across Europe, delivering life-saving medicines, medical products and healthcare services to patients wherever and whenever they need them. With more than 100 distribution centers across Europe, our infrastructure, capabilities and expertise have helped us successfully manage the storage and distribution of various COVID-19 vaccines according to their manufacturers’ requirements.
Additionally, a number of our McKesson-owned “banner” pharmacies have begun playing a vital part in national vaccine efforts throughout the UK. In late January, John Bell & Croyden pharmacy was chosen as McKesson’s first vaccination center in the UK and began vaccinating patients in London that same month. And in March, pharmacists at two LloydsPharmacy locations started their vaccination efforts in smaller local communities. As the vaccine rollout continues across Europe, more of our pharmacists will be involved in administering these injections.
Leading Canadian COVID-19 vaccination pilots, from distribution to pharmacy to patients
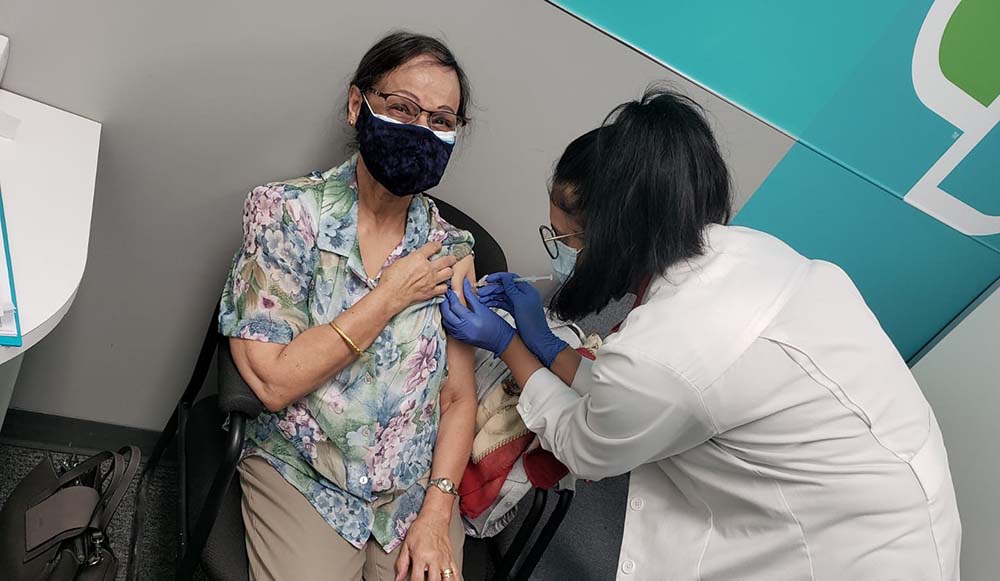
While the Canadian government is working to ensure everyone in Canada has access to COVID-19 vaccines as quickly as possible, the provinces and territories are individually responsible for planning and running their respective vaccination programs. This means that each region across Canada has a different approach to vaccinating their communities, which poses unique challenges for the country’s vaccination efforts. High patient-demand for vaccination, rapidly changing provincial roll-out plans, and uncertain volume from manufacturers all complicate the efforts. That’s why governments are relying on the support of healthcare companies like McKesson Canada to build the right system to safely and effectively vaccinate Canadians.
As a first step, several governments have launched vaccination pilot programs, or “test-runs,” starting in the provinces of Alberta, Ontario, Quebec, Nova Scotia, Manitoba and New Brunswick, with British Columbia, Saskatchewan and others expected to follow shortly. Included in these pilot programs, McKesson Canada’s corporately owned retail pharmacy chain, Rexall, delivered the first COVID-19 vaccines in community pharmacies in Alberta, marking a significant milestone in the ongoing efforts to stop the spread of COVID-19. All of these vaccines were distributed safely and proudly by McKesson Canada’s distribution team. McKesson Canada’s independent pharmacy banners, including Guardian, IDA, Remedy’sRx, The Medicine Shoppe, Uniprix and Proxim are also supporting Canada’s vaccination efforts.




Share
Post
Post
Email