Read time: 3 minutes
As an industry leader in vaccine distribution, McKesson is prepared to support the U.S. government through Operation Warp Speed to deliver Americans safe and effective COVID-19 vaccines as fast as possible. Earlier this year, the Centers for Disease Control and Prevention (CDC) designated our company as its centralized distribution partner for the U.S. COVID-19 Vaccination Program. And while this does not include the ultra-frozen Pfizer-BioNTech COVID-19 vaccine (the Pfizer vaccine), we will be distributing the Moderna vaccine and other future frozen and refrigerated vaccines once approved or authorized for emergency use by the U.S. Food and Drug Administration (FDA).
Yet, being ready to distribute vaccines is only part of the story. These vaccines also require ancillary supplies to administer them.
A few months ago, we partnered with the Strategic National Stockpile – part of the Office of the Assistant Secretary for Preparedness and Response within the U.S. Department of Health and Human Services – to assemble ancillary supply kits for the multiple COVID-19 vaccine candidates in large-scale (Phase 3) clinical trials. Ever since, we’ve been heavily engaged in preparing new distribution centers (DCs) and assembling a team to build and send out the supply kits.
And now that the FDA has issued the first Emergency Use Authorization (EUA) for the Pfizer vaccine, we’ve started to ship out ancillary supply kits through our carrier partners to point-of-care facilities throughout the country.
Providing a Sense of Scale

McKesson set up dedicated DCs to support ancillary supply kit assembly and storage for the U.S. government.
We added more than 1.5 million square feet of DC space for supply kit assembling and storage. For comparison, that’s about the size of Grand Central Station in New York City. And we used 3.7 million pounds of steel to create the supply kit holding racks in just one of our DCs – roughly one-fifth as heavy as the Eiffel Tower.
Recruiting a New Workforce

To support this monumental effort, we engaged several hundred workers in less than two months. With this new workforce in place, the supply kit distribution centers were fully operational within a matter of weeks.
Ready to Go

We’ve spent months preparing for the moment COVID-19 vaccine(s) become available. In fact, prior to the FDA’s EUA of the first COVID-19 vaccine, we put together enough supply kits to support administration of more than 100 million doses.
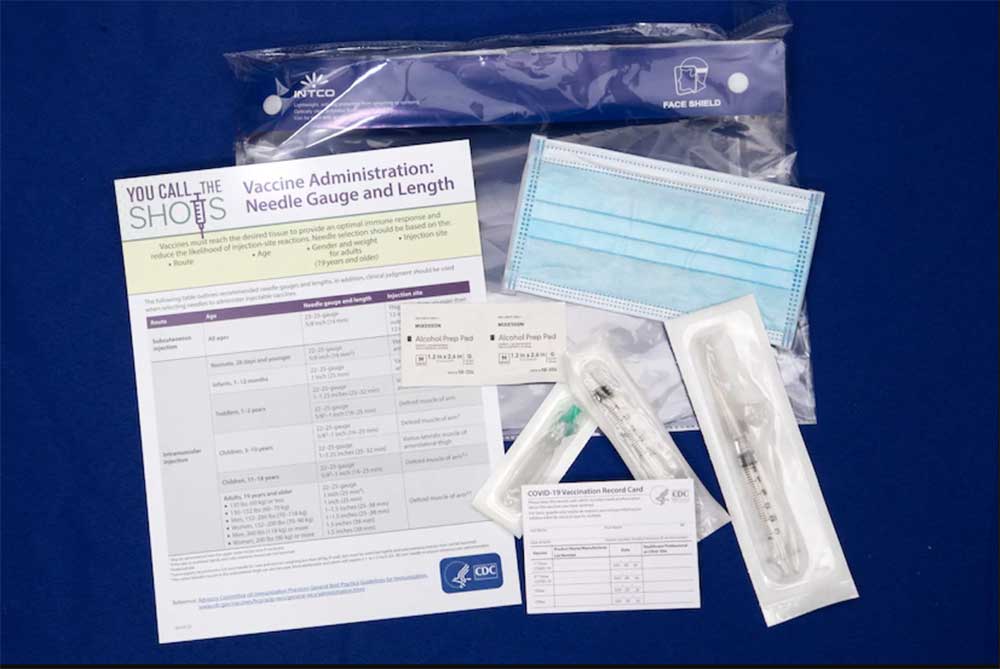
Each ancillary supply kit, except for the kits designated for use with the Pfizer vaccine, includes:
- Alcohol prep pads
- Surgical masks and face shields for vaccinators
- Needles
- Syringes
- Needle Gauge and Length Chart
- Vaccination record cards
Mixing kits are included in ancillary kits for the Pfizer vaccines that require reconstitution with diluent or mixing with adjuvant at the point of administration. The diluent is used to convert the ultra-frozen vaccine into a liquid form for administration.
The Details that Matter

For the refrigerated and frozen COVID-19 vaccines like Moderna’s, each kit includes the ancillary supplies needed to administer up to 100 doses of vaccine. The kits will usually be shipped at the same time and with the same carrier as the corresponding vaccine. For the ultra-frozen Pfizer COVID-19 vaccine, each supply kit supports 975 doses and is shipped using UPS.
Safety First

Protecting workers is crucial to the success of this critical effort. Safety measures are in place for everyone working at these McKesson DCs, which includes providing face masks and gloves, administering daily temperature checks, and maintaining social distancing. We also require regular use of hand sanitizer and cleaning wipes, which are located at stations throughout each DC.




Share
Post
Post
Email